Answer

1. Photophysical Properties of Molecules
Photophysical properties describe how molecules interact with light by absorbing, emitting, and dissipating energy. These interactions are key in spectroscopy, optoelectronics, and biological imaging.
Key Processes:
- Absorption: Photon promotes electron to excited state.
- Fluorescence: Fast emission from singlet excited state (S₁ → S₀).
- Phosphorescence: Slow emission from triplet state (T₁ → S₀).
- Internal Conversion: Non-radiative transition between states of same spin.
- Intersystem Crossing: Transition between singlet and triplet states.
- Non-Radiative Decay: Energy lost as heat without light emission.
Quantitative Parameters:
- Quantum Yield (Φ): Efficiency of photon emission.
- Lifetime (τ): Duration molecule remains excited.
- Stokes Shift: Energy/wavelength difference between absorption and emission maxima.
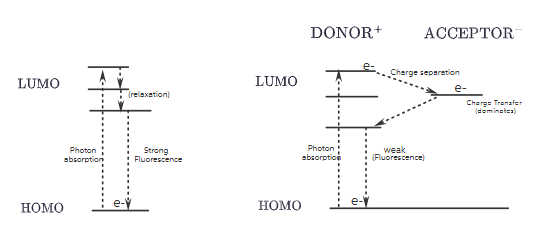
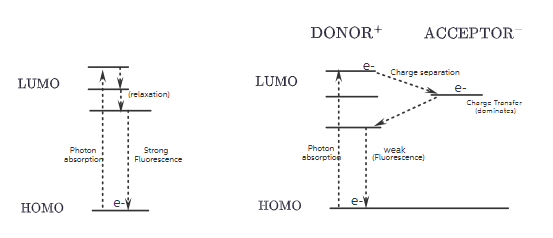
2. Molecular Orbital (MO) Diagrams
Sample 1 (Without Quencher)
- Ground State: Electron in HOMO.
- Excitation: Electron promoted to LUMO.
- Fluorescence: Electron returns to HOMO, emitting a photon.
Result: Strong fluorescence.
Dyad (Sample 1 + Covalently Linked Quencher)
- Same ground state as Sample 1.
- After excitation, electron transfers from donor’s LUMO to acceptor’s LUMO.
- Forms charge-separated state:
Donor⁺ — Acceptor⁻. - Competes with fluorescence, reducing its intensity and lifetime.
Result: Weaker fluorescence due to charge transfer dominance.
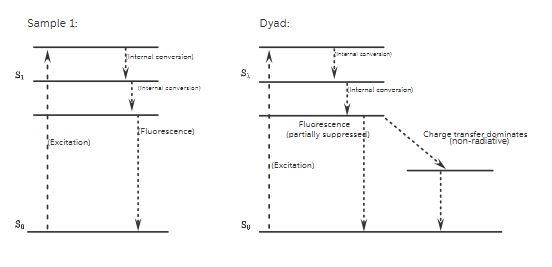
3. Jablonski Diagrams
Sample 1
- Excitation: S₀ → S₁ upon light absorption.
- Relaxation:
- Vibrational relaxation to S₁ minimum.
- Fluorescence: S₁ → S₀ radiative decay.
Result: Efficient fluorescence with no competing pathways.
Dyad
- Excitation: Donor excited from S₀ to S₁.
- Competing Processes:
- Charge Transfer (CT) from donor to acceptor suppresses fluorescence.
- Fluorescence partially occurs but with reduced intensity and faster decay.
- Possible Transitions:
- S₁ → CT state (non-radiative)
- CT → S₀ (slow recombination or quenching)
Result: Partially suppressed fluorescence with faster decay.
4. Conclusion
Without a quencher, molecules show strong fluorescence due to direct radiative relaxation. In the dyad system, fluorescence is diminished because of electron transfer to the acceptor, forming a charge-separated state. These principles are critical in designing photophysical systems for technologies like sensors, photovoltaics, and imaging.
